|
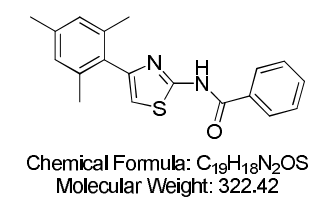
N-[4-(2,4,6-Trimethylphenyl)-2-thiazolyl]-benzamide
A cell-permeable INH1 analog exhibits significantly improved antiproliferative activity against MB231, MB468, HeLa, and K562 cells (IC50 = 1.7, 2.1, 2.4, and 2.5 µM, respectively)
OTAVAchemicals Catalogue Number: 7070707071
CAS Registry Number: 1001753-24-7
Purity: 97%+
Ref. 1: Qiu et al. Synthesis and Biological Evaluation of a Series of Novel Inhibitor of Nek2/Hec1 Analogues. Journal of Medicinal Chemistry (2009), 52, 1757-1767Ref. 1. Qiu et al. Synthesis and Biological Evaluation of a Series of Novel Inhibitor of Nek2/Hec1 Analogues. Journal of Medicinal Chemistry (2009), 52, 1757-1767;
Ref. 2: Zhu, Yongxia; Wei, Wei; Ye, Tinghong; Liu, Zhihao; Liu, Li; Luo, Yong; Zhang, Lidan; Gao, Chao; Wang, Ningyu; Yu, Luoting. Small Molecule TH-39 Potentially Targets Hec1/Nek2 Interaction and Exhibits Antitumor Efficacy in K562 Cells via G0/G1 Cell Cycle Arrest and Apoptosis Induction. Cellular Physiology and Biochemistry (2016), 40(1-2), 297-308;
Ref. 3: Ya. Rivera-Rivera et al. The Nek2 centrosome-mitotic kinase contributes to the mesenchymal state, cell invasion, and
migration of triple-negative breast cancer cells. Scientific Reports (2021), 11(1), 9016
Abstract 1. High expression in cancer 1 (Hec1) is an oncogene overly expressed in many human cancers. Small molecule inhibitor of Nek2/Hec1 (INH) targeting the Hec1 and its regulator, Nek2, in the mitotic pathway, was identified to inactivate Hec1/Nek2 function mediated by protein degradation that subsequently leads to chromosome mis-segregation and cell death. To further improve the efficacy of INH, a series of INH analogues were designed, synthesized, and evaluated. Among these 33 newly synthesized analogues, three of them, 6, 13, and 21, have 6-8 fold more potent cell killing activity than the previous lead compound INH1. Compounds 6 and 21 were chosen for analyzing the underlying action mechanism. They target directly the Hec1/Nek2 pathway and cause chromosome mis-alignment as well as cell death, a mechanism similar to that of INH1. This initial exploration of structural/functional relationship of INH may advance the progress for developing clinically applicable INH analogue.
Abstract 2. Cancer is still a major public health issue worldwide, and new therapeutics with anti-tumor activity are still urgently needed. Methods: The anti-tumor activity of TH-39, which shows potent anti-proliferative activity against K562 cells with an I C of 0.78 ìM, was investigated using immunoblot, co-immunoprecipitation, the MTT assay, and flow cytometry. Results: Mechanis tically, TH-39 may disrupt the interaction between Hec1 and Nek2 in K562 cells. Moreover, T H-39 inhibited cell proliferation in a concent ration- and time-dependent manner by influencing the morphol. of K562 cells and inducing G0/G1 phase arrest. G0/G1 phase arrest was associated with down-regulation of CDK2-cyclin E complex and CDK4/6-cyclin D complex activities. Furthermore, TH-39 also induced cell apoptosis, which was associated with activation of caspase-3, down-regulation of Bcl-2 expression and up-regulation of Bax. TH-39 could also decrease mitochondrial membrane potential (Äøm) and increase reactive oxygen species (R OS) accumulation in K562 cells. The results indicated that TH-39 might induce apoptosis via the R OS-mitochondrial apoptotic pathway. Conclusion:
This study highlights the potential therapeutic efficacy of the anti-cancer compound TH-39 in treatment-resistant chronic myeloid leukemia.
Abstract 3. Nek2 (NIMA-related kinase 2) is a serine/threonine-protein kinase that localizes to centrosomes and kinetochores, controlling centrosome separation, chromosome attachments to kinetochores, and the spindle assembly checkpoint. These processes prevent centrosome amplification (CA), mitotic dysfunction, and chromosome instability (CIN). Our group and others have suggested that Nek2 maintains high levels of CA/CIN, tumor growth, and drug resist ance. We identified that Nek2 overexp ression correlates with poor survival of breast cancer. However, the mechanisms driving these phenotypes are unknown. We now report that overexp ression of Nek2 in MCF10A cells drives CA/CIN and aneuploidy. Besides, enhanced levels of Nek2 results in larger 3 D acinar struct ures, but could not initiate tumors in a p53+/+ or a p53- /- xenograft model. Nek2 overexp ression induced the epithelial-to-mesenc hymal transition (EMT) while its downregulation reduced the expression of the mesenchymal marker vimentin. Furthermore, either siRNA-mediated downregulation or INH6's chem. inhibition of Nek2 in M DA-MB-231 and Hs578t cells showed important E MT changes and decreased invasion and migration. We also showed that Slug and Zeb1 are involved in Nek2 mediated EMT, invasion, and migration. Besides its role in CA/CIN, Nek2 contributes to breast cancer progression through a novel EMT mediated mechanism.
DOI:
10.1021/jm8015969
10.1159/000452546
10.1038/s41598-021-88512-0
Price info:
|
1 MG |
59 EUR |
|
5 MG |
99 EUR |
|
10 MG |
129 EUR |
|
300uL of 10mM solution |
79 EUR |
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS

