|
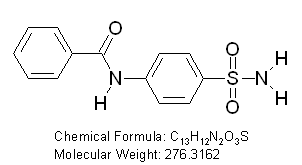
N-(4-sulfamoylphenyl)benzamide
exhibited promising anticonvulsant effect against MES model for inhibition of Lyase- Human Carbonic Anhydrase-I
OTAVAchemicals Catalogue Number: 0127441873
CAS Registry Number: 4389-07-5
Purity: 95%
Ref.: Ajeet, Kumar, A., & Mishra, A. K. (2018). Design, molecular docking, synthesis, characterization, biological activity evaluation (against MES model), in-silico biological activity spectrum (PASS analysis), toxicological and predicted oral rat LD50 studies of novel sulphonamide derivatives. Frontiers in Biology
Abstract: BACKGROUND: Among the reported potential agents to treat the epilepsy, sulphonamides are important and their significance cannot be ignored. A series of substituted 4-amino-benzene sulfonamides were designed, keeping in view the structural requirement of pharmacophore.
METHODS: Lipinski rule of five has been calculated; failure to Lipinski rule was not observed. Docking was performed through AutoDock Vina. Molecules have been screened out through docking. Compounds were synthesized and characterized through IR, 1
HNMR, 13C NMR, Mass and elemental analysis. The anticonvulsant activity of the synthesized compounds was assessed using the Maximal Electroshock Seizure (MES) model. In-silico biological activity spectrum, toxicological studies, predicted oral rats LD50 were performed.
RESULTS: Docking studies showed good interaction with lyase (Oxo-acid) - human carbonic anhydrase-I (1AZM). The insilico studies proved them to be with good drug-likeness properties, especially 4-(3-Acetyl-phenylamino)-methyl)-benzenesulfonamide (2g). These results revealed that the synthesized compounds (1a-1c, 2a-2q) exhibited promising anticonvulsant effect against MES model for inhibition of Lyase- Human Carbonic Anhydrase-I.
CONCLUSION: After investigating all the results, the compound 4-(3-Acetyl-phenylamino)-methyl)-benzenesulfonamide (2g) is found to be best in the series. A comparatively good activity of compound 2g suggests us that sulphonamide can be leads to further optimization for building potent and chemically diversified anti-convulsant agents.
DOI: 10.1007/s11515-018-1512-4
Price info:
|
1 MG |
35 EUR |
|
5 MG |
49 EUR |
|
10 MG |
59 EUR |
|
300uL of 10mM solution |
45 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS

