|
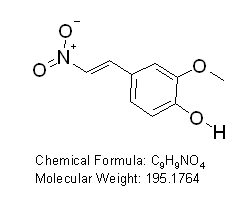
2-methoxy-4-[(E)-2-nitrovinyl]phenol
Potential antibacterial agent against the Gram-positive bacteria (MIC = 256 mg/L)
OTAVAchemicals Catalogue Number: 0107930017
CAS Registry Number: 6178-42-3
Purity: 95%
Ref.: b-Nitrostyrene derivatives as potential antibacterial agents: A structure–property–activity relationship study. Milhazes, N., Calheiros, R., Marques, M. P. M., Garrido, J., Cordeiro, M. N. D. S., Rodrigues, C., Borges, F. Bioorg. Med. Chem., 2006, 14, 4078–4088.
Abstract: A multidisciplinary project was developed, combining the synthesis of a series of b-nitrostyrene derivatives and the determination of their physicochemical parameters (redox potentials, partition coefficients), to the evaluation of the corresponding antibacterial activity. A complete conformational analysis was also performed, in order to get relevant structural information. Subsequently, a structure–property–activity (SPAR) approach was applied, through linear regression analysis, aiming at obtaining a putative correlation between the physicochemical parameters of the compounds investigated and their antibacterial activity (both against standard strains and clinical isolates). The b-nitrostyrene compounds displayed a lower activity towards all the tested bacteria relative to the b-methyl-b-nitrostyrene analogues. This was observed particularly for the 3-hydroxy-4-methoxy-b-methyl-bnitrostyrene (IVb) against the Gram-positive bacteria (Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium). The SPAR results revealed the existence of a clear correlation between the redox potentials and the antibacterial activity of the series of b-nitrostyrene derivatives under study.
DOI: 10.1016/j.bmc.2006.02.006
Price info:
|
1 MG |
35 EUR |
|
5 MG |
49 EUR |
|
10 MG |
69 EUR |
|
300uL of 10mM solution |
39 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS

