|
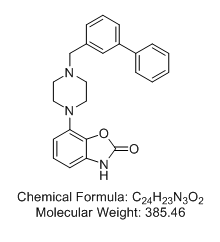
7-[4-([1,1'-Biphenyl]-3-ylmethyl)-1-piperazinyl]-2(3H)-benzoxazolone (Bifeprunox)
a potent dopamine D2-like and 5-HT1A receptor partial agonist with pKis of 7.19 and 8.83 for cortex 5-HT1A and striatum D2, and a pEC50 of 6.37 for hippocampus 5-HT1A, respectively.
Bifeprunox is an antipsychotic for the research of schizophrenia
OTAVAchemicals Catalogue Number: 7070707030
CAS Registry Number: 350992-10-8
Purity: 97%+ (HPLC)
Ref. 1: Feenstra et al. New 1-aryl-4-(biarylmethylene)piperazines as potential atypical antipsychotics sharing dopamine D2-receptor and serotonin 5-HT1A-receptor affinities. Bioorganic & Medicinal Chemistry Letters (2001), 11, 2345-2349
Abstract: 1-Aryl-4-(biarylmethylene)piperazines were prepared and their affinity for D2 and 5-HT1A receptors was determined. A selection of these compounds was evaluated in vivo, resulting in the identification of a drug candidate Bifeprunox which is being clinically evaluated as a potential atypical antipsychotic with reduced extrapyrimidal side effects.
Ref. 2: Watanabe, Mark D. Bifeprunox. A partial dopamine-receptor agonist for the treatment of schizophrenia. Formulary (2007), 42, 371-377
Abstract: Schizophrenia is a chronic psychiatric disorder that affects an estimated 1% of the population. This disorder may be treated with typical (first-generation) or atypical (second-generation) agents; a recognized concern regarding these agents is that long-term use has been associated with increased risks of serious side effects, either neurologic or metabolic in nature. Bifeprunox is a partial dopamine-receptor agonist under investigation for the treatment of patients with schizophrenia. As a partial dopamine-receptor agonist, bifeprunox acts as a dopamine-system stabilizer. This proposed mechanism of action is similar to that of aripiprazole but different from that of the other currently marketed antipsychotic medications. Available clinical and safety data are limited but describe positive effects in treating acute psychotic symptoms and prolonging time to deterioration, with a generally tolerable side-effect profile. If approved, bifeprunox may serve as an additional option for the acute and maintenance treatment of schizophrenia.
Ref. 3: Newman-Tancredi et al. Neuropharmacological profile of bifeprunox: merits and limitations in comparison with other third-generation antipsychotics. Current Opinion in Investigational Drugs (Thomson Scientific) (2007), 8, 539-554
A review. Schizophrenia is characterized by a range of positive and negative symptoms, and cognitive deficits. While positive symptoms respond to current antipsychotic agents, negative symptoms and cognitive deficits are often resistant to pharmacopea. Thus research is now focused on developing third-generation antipsychotics that combine antagonism or partial agonism at dopamine D(2)-like receptors with agonism at serotonin 5-HT(1A) receptors. Such an association is anticipated to provide therapeutic benefits against a broader range of schizophrenia symptoms. Bifeprunox is one such third-generation antipsychotic agent which acts as a partial agonist at D(2)-like receptors and is an efficacious agonist at 5-HT(1A) receptors, with little interaction at 5HT(2A/2C), muscarinic or histaminergic H(1) receptors. This review summarizes the pharmacological profiles of the current antipsychotic agents and describes the rationale behind the development of third-generation antipsychotics. It also evaluates current data concerning bifeprunox in comparison with currently available antipsychotics, as well as those that are still under clinical development.
DOI: 10.1016/s0960-894x(01)00425-5
Price info:
|
1 MG |
59 EUR |
|
5 MG |
99 EUR |
|
10 MG |
129 EUR |
|
300uL of 10mM solution |
79 EUR |
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


