|
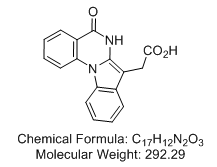
5,6-Dihydro-5-oxo-indolo[1,2-a]quinazoline-7-acetic acid
potent and selective CK2 inhibitor; Ki = 0.17 µM
OTAVAchemicals Catalogue Number: 7020402316
CAS Registry Number: 391670-48-7
Purity: 95%+ (HPLC)
Ref. 1: Vangrevelinghe et al. Discovery of a Potent and Selective Protein Kinase CK2 Inhibitor by High-Throughput Docking. Journal of Medicinal Chemistry (2003), 46, 2656-2662
Abstract: To assess the potential of protein kinase CK2 as a target for developing new antitumor agents, we have undertaken a search for inhibitors of this enzyme. As part of this effort, we report here the discovery of the potent and selective CK2 inhibitor (5-oxo-5,6-dihydroindolo[1,2-a]-quinazolin-7-yl)acetic acid. We identified this inhibitor of a novel structural type by highthroughput docking of our corporate compound collection in the ATP binding site of a homology model of human CK2, using an appropriate protocol. The synthesis of the inhibitor as well as that of related analogues whose CK2 inhibitory activities give support to the binding mode proposed by the docking program is described. The results obtained suggest that virtual screening of a 3D database by molecular docking is a useful approach for lead finding provided that adapted post-docking filtering and reranking procedures are applied to the primary hit list.
Ref. 2: Sarno et al. Development and exploitation of CK2 inhibitors. Molecular and Cellular Biochemistry (2005), 274, 69-76
Abstract: A no. of quite specific and fairly potent inhibitors of protein kinase CK2, belonging to the classes of condensed polyphenolic compds., tetrabromobenzimidazole/triazole derivatives and indoloquinazolines are available to date. The structural basis for their selectivity is provided by a hydrophobic pocket adjacent to the ATP/GTP binding site, which in CK2 is smaller than in the majority of other protein kinases due to the presence of a no. of residues whose bulky side chains are generally replaced by smaller ones. Consequently a doubly substituted CK2 mutant V66A,I174A is much less sensitive than CK2 wild type to these classes of inhibitors. The most efficient inhibitors both in terms of potency and selectivity are 4,5,6,7-tetrabromo-1H-benzotriazole, TBB (Ki = 0.4 μM), the TBB derivative 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole, DMAT (Ki = 0.040 μM), the emodin related coumarinic compound 8-hydroxy-4-methyl-9-nitrobenzo[g]chromen-2-one, NBC (Ki = 0.22 μM) and the indoloquinazoline derivative ([5-oxo-5,6-dihydroindolo-(1,2a)quinazolin-7-yl]acetic acid), IQA (Ki = 0.17 μM). These inhibitors are cell permeable as judged from ability to block CK2 in living cells and they have been successfully employed, either alone or in combination with CK2 mutants refractory to inhibition, to dissect signaling pathways affected by CK2 and to identify the endogenous substrates of this pleiotropic kinase. By blocking CK2 these inhibitors display a remarkable pro-apoptotic efficacy on a no. of tumor derived cell lines, a property which can be exploited in perspective to develop antineoplastic drugs.
DOI:
10.1021/jm030827e
10.1007/s11010-005-3079-z
Price info:
|
1 MG |
79 EUR |
|
5 MG |
99 EUR |
|
10 MG |
129 EUR |
|
300uL of 10mM solution |
89 EUR |
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS

