|
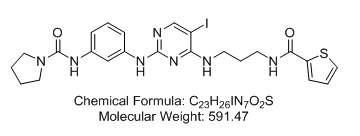
N-[3-[[5-Iodo-4-[[3-[(2-thienylcarbonyl)amino]propyl]amino]-2-pyrimidinyl]amino]phenyl]-1-pyrrolidinecarboxamide
highlighted BX 795 bioactivitiy as a potent and relatively specific inhibitor of TBK1 and closely related IKKe, with IC50 values to be 6, 41, and 111 nM for TBK1, IKKe and PDK1 respectively
OTAVAchemicals Catalogue Number: 7070707044
CAS Registry Number: 702675-74-9
Purity: 95%+ (HPLC)
Ref.:Cohen et al. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkB kinase: a distinct upstream kinase mediates SER-172 phosphoylation and activation. Journal of Biological Chemistry (2009), 284, 14136-14146
Abstract:TANK-binding kinase 1 (TBK1) and IkB kinase ϵ(IKKϵ) regulate the production of Type 1 interferons during bacterial and viral infection, but the lack of useful pharmacological inhibitors has hampered progress in identifying additional physiological roles of these protein kinases and how they are regulated. Here we demonstrate that BX795, a potent and relatively specific inhibitor of TBK1 and IKKϵ, blocked the phosphorylation, nuclear translocation, and transcriptional activity of interferon regulatory factor 3 and, hence, the production of interferon-beta in macrophages stimulated with poly(I:C) or lipopolysaccharide (LPS). In contrast, BX795 had no effect on the canonical NFkB signaling pathway. Although BX795 blocked the autophosphorylation of overexpressed TBK1 and IKKe at Ser-172 and, hence, the autoactivation of these protein kinases, it did not inhibit the phosphorylation of endogenous TBK1 and IKKe at Ser-172 in response to LPS, poly(I:C), interleukin-1alpha (IL-alpha), or tumor necrosis factoralpha and actually enhanced the LPS, poly(I:C), and IL-1 alpha stimulated phosphorylation of this residue. These results demonstrate that the phosphorylation of Ser-172 and the activation of TBK1 and IKKe are catalyzed by a distinct protein kinase(s) in vivo and that TBK1 and IKKe control a feedback loop that limits their activation by LPS, poly(I:C) and IL-1alpha (but not tumor necrosis factor alpha) to prevent the hyperactivation of these enzymes.
DOI:10.1074/jbc.M109.000414
Price info:
|
1 MG |
35 EUR |
|
5 MG |
80 EUR |
|
10 MG |
130 EUR |
|
300uL of 10mM solution |
49 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


