|
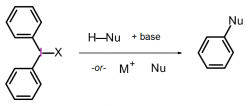
 Diaryliodonium salts are well-known to transfer an aryl group to carbon and heteroatom nucleophiles, and in some cases a base is required [1]. However, transition metal catalysts and supporting ligands are not needed. Moreover, reactions conducted with diaryliodonium salts are operationally simple because they are non-toxic and are not sensitive to air or moisture. Therefore diaryliodonium salts offer an important alternative to metal-catalyzed arylation reactions. Despite these attractive characteristics, a major obstacle to their adoption in chemical synthesis and discovery chemistry has been commercial availability. Diaryliodonium salts are well-known to transfer an aryl group to carbon and heteroatom nucleophiles, and in some cases a base is required [1]. However, transition metal catalysts and supporting ligands are not needed. Moreover, reactions conducted with diaryliodonium salts are operationally simple because they are non-toxic and are not sensitive to air or moisture. Therefore diaryliodonium salts offer an important alternative to metal-catalyzed arylation reactions. Despite these attractive characteristics, a major obstacle to their adoption in chemical synthesis and discovery chemistry has been commercial availability.
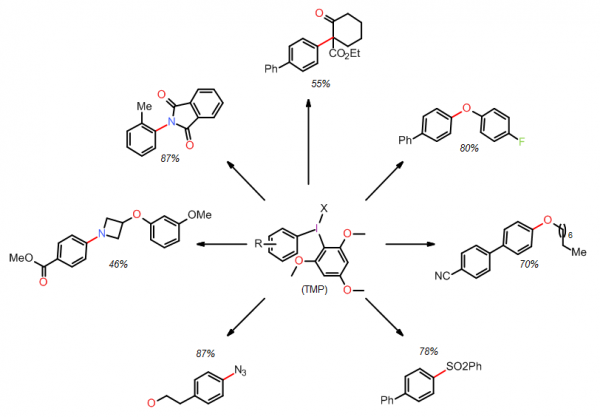
In collaboration with the Stuart Group at Portland State University, we now offer > 60 structurally distinct unsymmetrical diaryliodonium salts bearing a 2,4,6-trimethoxyphenyl (TMP) auxiliary that serves as a non-transferable “dummy” group. The aryl groups of the aryl(TMP)iodonium tosylates feature a diverse range of substituents, such as hydrocarbyl, halides, trifluoromethyl, nitro, nitrile, ester, amide, phenyl ether, hydroxyl(methyl), bromo(methyl), and substitution patterns. The use of unsymmetrical aryl(TMP)iodonium salts allow for exclusive aryl transfer selectivity and facilitates purification by easy removal of the iodo-2,4,6-trimethoxyphenyl group. Although method development for metal-free arylation reactions is continually expanding, the Stuart group has already demonstrated that they react with malonate, phenoxide and alkoxide, phenyl sulfinate, and azide, imide, and alicyclic amine nucleophiles [2, 3, 4, 5]. Others have also demonstrated that aryl(TMP)iodonium salts are useful reagents in radical arylations with visible light photoredox catalysis or under basic conditions thus diversifying the reactivity profile of these reagents [6, 7].
References:
-
Olofsson B. Arylation with Diaryliodonium Salts. Topics in Current Chemistry (in Hypervalent Iodine Chemistry). 2016, Vol. 373, pp. 135-166.
-
Seidl T. L., Sundalam S. K., McCullough B., Stuart D. R. Unsymmetrical Aryl(2,4,6-trimethoxyphenyl)iodonium Salts: One-pot Synthesis, Scope, Stability, and Synthetic Studies. Journal of Organic Chemistry. 2016, Vol. 81, pp. 1998 – 2009.
-
Sandtorv A. H., Stuart D. R. Metal-free Synthesis of Aryl Amines: Beyond Nucleophilic Aromatic Substitution. Angewandte Chemie International Edition. 2016, Vol. 55, pp. 15812-15815.
-
Seidl T. L., Stuart D. R. An Admix Approach to Determine Counter Anion Effects on Metal-Free Arylation Reactions with Diaryliodonium Salts. Journal of Organic Chemistry. 2017, Vol. 82, pp. 11765-11771.
-
Basu S., Sandtorv A. H., Stuart D. R. Imide Arylation with Aryl(TMP)iodonium Tosylates. Beilstein Journal of Organic Chemistry. 2018, Vol. 14, pp. 1034-1038.
-
Dohi T., Ueda S., Hirai A., Kojima Y., Morimoto K., Kita Y. Selective Aryl Radical Transfers into Heteroaromatics from Diaryliodonium Salts with Trimethoxybenzene Auxiliary. Heterocycles. 2017, Vol. 95, pp. 1272-1284.
-
Sun D., Yin K., Zhang R. Visible-Light-Induced Multicomponent Cascade Cycloaddition Involving N-Propargyl Aromatic Amines, Diaryliodonium Salts, and Sulfur Dioxide: Rapid Access to 3-Arylsulfonylquinolines. Chemical Communications. 2018, Vol. 54, pp. 1335-1338.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS




