|
EGFR (HER-1 in humans) Inhibitor |
|
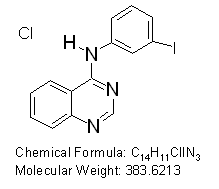
N-(3-iodophenyl)-4-quinazolinamine hydrochloride
Potent inhibitor of Epidermal growth factor receptor erbB1 with IC50 = 80 nM.
OTAVAchemicals Catalogue Number: 7017500003
CAS Registry Number: N/A
Purity: 98%
Ref.: Rewcastle et al. Tyrosine Kinase Inhibitors. 5. Synthesis and Structure-Activity Relationships for 4-[(Phenylmethyl)amino]- and 4-(Phenylamino)quinazolines as Potent Adenosine 5'-Triphosphate Binding Site Inhibitors of the Tyrosine Kinase Domain of the Epidermal Growth Factor Receptor J. Med. Chem. (1996), 38, 3482-3487
Abstract: A series of 4-substituted quinazolines and related compounds have been prepared and evaluated for their ability to inhibit the tyrosine kinase activity of the epidermal growth factor receptor on a phospholipase C-gamma 1-derived substrate. The results show a narrow structure-activity relationship (SAR) for the basic ring system, with quinazoline being the preferred chromophore and benzylamino and anilino the preferred side chains. In the 4-anilino series, substitution on the 3-position of the phenyl ring with small lipophilic electron-withdrawing groups provided analogues with enhanced potency. Two series of compounds [4-(phenylmethyl)amino and 4-(3-bromophenyl)amino] were studied to determine SAFb for quinazoline substituents. In the more active 4-(3-bromophenyl)amino series, electron-donating groups (NH2, OMe) at the 6- or 7-position increased activity, in a pattern consistent with a requirement for high electron density in the vicinity of the 8-position of the quinazoline ring. The 6,7-dimethoxy derivatives were the most effective in both series, with the 4-(3-bromophenyl)amino derivative (3) having an IC50 of 0.029 nM, making it by far the most potent reported inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor enzyme.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


