|
Daidzein (7-Hydroxy-3-(4-hydroxyphenyl) chromen-4-one)
Potent inhibitor of monoamine oxidase (MAO) and aldehyde dehydrogenase (ALDH-2)
with IC50 = 14 μM and 9 μM, respectively.
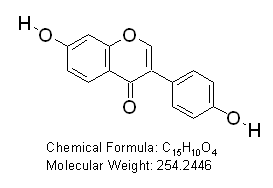
OTAVAchemicals Catalogue Number: 7119860038
CAS Registry Number: 486-66-8
Purity: 99%
Ref. 1: Gao et al. Synthesis of Potential Antidipsotropic Isoflavones: Inhibitors of the Mitochondrial Monoamine Oxidase Aldehyde Dehydrogenase Pathway. J. Med. Chem. 2001, 44, 3320-3328.
Abstract: Recently we have shown that daidzin, the major active principle of an ancient herbal treatment for “alcohol addiction”, suppresses ethanol intake in alcohol-preferring laboratory animals. Further, we have identified the monoamine oxidase (MAO) aldehyde dehydrogenase (ALDH-2) pathway of the mitochondria as the potential site of action of daidzin. Daidzin analogues that potently inhibit ALDH-2 but have no or little effect on MAO are most antidipsotropic, whereas those that also inhibit MAO exhibit little, if any, antidipsotropic activity. Therefore, in the design and synthesis of more potent antidipsotropic analogues, structural features important for the inhibition of both ALDH-2 and MAO must be taken into consideration. To gain further information on the structure activity relationships at the inhibitor binding sites of ALDH-2 and MAO, we prepared 44 analogues of daidzin and determined their potencies for ALDH-2 and MAO inhibition. Results indicate that a sufficient set of criteria for a potent antidipsotropic analogue is an isoflavone with a free 4′-OH function and a straight-chain alkyl substituent at the 7 position that has a terminal polar function such as OH, COOH, or NH2. The preferable chain lengths for the 7-O-ω-hydroxy, 7-O-ω-carboxy, and 7-O-ω-amino subsitutents are 2 ≤ n ≤ 6, 5 ≤ n ≤ 10, and n ≥ 4, respectively. Analogues that meet these criteria have increased potency for ALDH-2 inhibition and/or decreased potency for MAO inhibition and therefore are likely to be potent antidipsotropic agents.
Ref.2: H. Matsuda et al. Phytoestrogens from the Roots of Polygonum cuspidatum (Polygonaceae): Structure-Requirement of Hydroxyanthraquinones for Estrogenic Activity. Bioorg. Med. Chem. Lett. 11 (2001), 1839–1842.
Abstract: The methanolic extract from the roots of Polygonum (P.) cuspidatum was found to enhance cell proliferation at 30 or 100 mg/mL in MCF-7, an estrogen-sensitive cell line. By bioassay-guided separation from P. cuspidatum with the most potent activity, emodin and emodin 8-O-β-d-glucopyranoside were isolated as active principles. The methanolic extracts from Polygonum, Cassia, Aloe, and Rheum species, which were known to contain anthraquinones, also showed the MCF-7 proliferation. As a result of the evaluation of various anthraquinones from plant sources and synthetic anthraquinones, aloe-emodin, chrysophanol, chrysophanol 8-O-β-d-glucopyranoside, and 1,8-dihydroxyanthraquinone showed weak activity. On the other hand, alizalin and 2,6-dihydroxyanthraquinone as well as emodin having the 2- and/or 6-hydoxyl groups showed potent activity. These results show that the unchelated hydroxyl group is essential for strong activity. Emodin and 2,6-dihydroxyanthraquinone also inhibited 17b-estradiol binding to human estrogen receptors (ERs) with Ki values of 0.77 and 0.31 mM for ERa and 1.5 and 0.69 mM for ERb. These findings indicate that hydroxyanthraquinones such as emodin are phytoestrogens with an affinity to human estrogen receptors.
Ref.3: Jiang et al. Effects of 7-O Substitutions on Estrogenic and Antiestrogenic Activities of Daidzein Analogues in MCF-7 Breast Cancer Cells. J Med Chem. 53(16), (2010) 6153–6163.
Abstract: Daidzein (1) is a natural estrogenic isoflavone. We report here that 1 can be transformed into antiestrogenic ligands by simple alkyl substitutions of the 7-hydroxyl hydrogen. To test the effect of such structural modifications on the hormonal activities of the resulting compounds, a series of daidzein analogues have been designed and synthesized. When MCF-7 cells were treated with the analogues, those resulting from hydrogen substitution by isopropyl (3d), isobutyl (3f), cyclopentyl (3g), and pyrano- (2), inhibited cell proliferation, estrogen-induced transcriptional activity, and estrogen receptor (ER) regulated progesterone receptor (PgR) gene expression. However, methyl (3a) and ethyl (3b) substitutions of the hydroxyl proton only led to moderate reduction of the estrogenic activities. These results demonstrated the structural requirements for the transformation of daidzein from an ER agonist to an antagonist. The most effective analogue, 2 was found to reduce in vivo estrogen stimulated MCF-7 cell tumorigenesis using a xenograft mouse model.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


