|
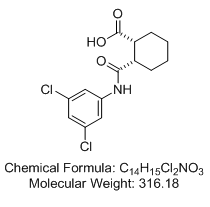
(±)-cis-2-{[(3,5-dichlorophenyl)amino]carbonyl}cyclohexanecarboxylic acid ((1R,2S)-rel-2-[[(3,5-dichlorophenyl)amino]carbonyl]-cyclohexanecarboxylic acid; VU0155041)
• Positive allosteric selective modulator at mGluR4 (EC50 values are 798 nM and 693 nM at human and rat mGluR4 receptors respectively). It has advantages over PHCCC (Asc-043) as it is water soluble and more potent. Active in behavioural models of Parkinsons Disease
Relative stereochemistry
OTAVAchemicals Catalogue Number: 7114471128
CAS Registry Number: 1093757-42-6
CAS Registry Number: 1259372-69-4 (sodium salt)
Purity: 95%+ (HPLC)
Ref.: Niswender et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Molecular Pharmacology (2008), 74, 1345-1358
Abstract: Parkinson's disease (PD) is caused by the death of dopamine neurons in the basal ganglia and results in motor symptoms such as tremor and bradykinesia. Activation of metabotropic glutamate receptor 4 (mGluR4) has been shown to modulate neurotransmission in the basal ganglia and results in antiparkinsonian effects in rodent PD models. N-Phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) is a positive allosteric modulator (PAM) of mGluR4 that has been used to further validate the role of mGluR4 in PD, but the compound suffers from a lack of selectivity, relatively low potency, and poor solubility. Via high-throughput screening, we discovered more than 400 novel PAMs of mGluR4. Compounds derived from a novel chemical scaffold were characterized in vitro at both rat and human mGluR4 using two distinct assays of mGluR4 function. The lead compound was approximately 8-fold more potent than PHCCC, enhanced the potency of glutamate at mGluR4 by 8-fold, and did not show any significant potentiator or antagonist activity at other mGluR subtypes. Resolution of the regioisomers of the lead revealed that the cis regioisomer, (±)-cis-2-(3,5-dichlorphenylcarbamoyl)cyclohexanecarboxylic acid (VU0155041), contained the majority of the mGluR4 PAM activity and also exhibited partial agonist activity at mGluR4 at a site that was distinct from the glutamate binding site, suggesting that this compound is a mixed allosteric agonist/PAM of mGluR4. VU0155041 was soluble in an aqueous vehicle, and intracerebroventricular administration of 31 to 316 nmol of VU0155041 dose-dependently decreased haloperidol-induced catalepsy and reserpine-induced akinesia in rats. These exciting results provide continued support for mGluR4 as a therapeutic target in PD.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


