|
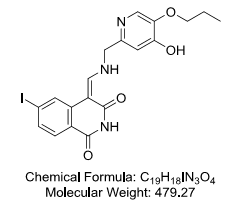
4-[[[(4-Hydroxy-5-propoxy-2-pyridinyl)methyl]amino]methylene]-6-iodo-1,3(2H,4H)-isoquinolinedione
Potent and selective inhibitors of the Cyclin-Dependent Kinase 4
OTAVAchemicals Catalogue Number: 7070707068
CAS Registry Number: 943746-57-4
Purity: 97%
Ref.: Tsou et al. Discovery of 4-(Benzylaminomethylene)isoquinoline-1,3-(2H,4H)-diones and 4-[(Pyridylmethyl)aminomethylene]isoquinoline-1,3-(2H,4H)-diones as Potent and Selective Inhibitors of the Cyclin-Dependent Kinase 4. Journal of Medicinal Chemistry (2009), 52, 2289-2310
Abstract: The series of 4-(benzylaminomethylene)isoquinoline-1,3-(2H,4H)-dione and 4-[(pyridylmethyl)aminomethylene]isoquinoline-1,3-(2H,4H)-dione derivatives reported here represents a novel class of potential antitumor agents, which potently and selectively inhibit CDK4 over CDK2 and CDK1. In the benzylamino headpiece, a 3-OH substituent is required on the phenyl ring for CDK4 inhibitory activity, which is further enhanced when an iodo, aryl, heteroaryl, t-butyl, or cyclopentyl substituent is introduced at the C-6 position of the isoquinoline-1,3-dione core. To circumvent the metabolic liability associated with the phenolic OH group on the 4-substituted 3-OH phenyl headpiece, we take two approaches: first, introduce a nitrogen o- or p- to the 3-OH group in the phenyl ring; second, replace the phenyl headpiece with N-substituted 2-pyridones. We present here the synthesis, SAR data, metabolic stability data, and a CDK4 mimic model that explains the binding, potency, and selectivity of our CDK4 selective inhibitors.
DOI: 10.1021/jm801026e
Price info:
|
1 MG |
59 EUR |
|
5 MG |
150 EUR |
|
10 MG |
199 EUR |
|
300uL of 10mM solution |
70 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


