|
7-hydroxy-5-methyl-2-(2-oxopropyl)-4H-chromen-4-one
An inhibitor of the production of nitric oxide (NO)
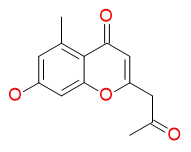
Chemical Formula: C13H12O4
Molecular Weight: 232.2382
OTAVAchemicals Catalogue Number: 3699690
CAS Registry Number: 40738-40-7
Purity: 95% (HPLC)
Ref.: Liu, Q., Mu, Y., An, Q., Xun, J., Ma, J., Wu, W., … Huang, X. (2019). Total synthesis and anti-inflammatory evaluation of violacin A and its analogues. Bioorganic Chemistry, 103420.
Abstract: A concise total synthesis of an exceedingly potent anti-inflammatory agent violacin A as well as the preparation of thirty analogues of this lead from commercially available orcinol are described. Highlights of our synthetic efforts involve Friedel-Crafts acylation, the regioselective etherification and esterification of phenolic hydroxyl groups, and Baker-Venkatamaran rearrangement to form basic skeleton of violacin A. The deprotection reaction with Pd-catalytic was involved to avoid the elimination of the hemiacetal hydroxyl at C2. In addition, all synthetic compounds were screened for anti-inflammatory activity against nitric oxide (NO) production using lipopolysaccharide (LPS)-induced Raw264.7 cells. A range of violacin A derivatives 11b, 11d, 11f, 12e, 12g, 13g, 17d-g exhibited stronger anti-inflammatory effect than that of violacin A. Notably, halogeno-benzyloxy substituent at C-7 were favourable for anti-inflammatory activities of violacin A derivatives. Additionally, Western blot results indicated halogeno-benzyloxy derivatives inhibited pro-inflammatory cytokines releases correlated with the suppression of NF-ƒÛB signaling pathway.
DOI: 10.1016/j.bioorg.2019.103420
Price info:
|
1 MG |
79 EUR |
|
5 MG |
129 EUR |
|
10 MG |
199 EUR |
|
300uL of 10mM solution |
104 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


