|
4-[5-(4-Chlorophenyl)-2-methyl-3-(1-oxopropyl)-1H-pyrrol-1-yl]benzenesulfonamide
Positive allosteric modulator of a7 nAChRs
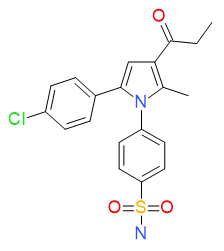
Chemical Formula: C20H19ClN2O3S
Molecular Weight: 402.9
OTAVAchemicals Catalogue Number: 7070707121
CAS Registry Number: 1000279-69-5
Purity: 98% (HPLC)
Ref. 1: Malysz, J., Grønlien, J.H., Anderson, D.J., et al. In vitro pharmacological characterization of a novel allosteric modulator of a7 neuronal acetylcholine receptor, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744), exhibiting unique pharmacological profile. J. Pharmacol. Exp. Ther. 330(1), 257-267 (2009).
Ref. 2: Faghih, R., Gopalakrishnan, S.M., Gronlien, J.H., et al. Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the a7 nicotinic acetylcholine receptor. J. Med. Chem. 52(10), 3377-3384 (2009).
Abstract: Targeting a7 neuronal acetylcholine receptors (nAChRs) with selective agonists and positive allosteric modulators (PAMs) is considered a therapeutic approach for managing cognitive deficits in schizophrenia and Alzheimer's disease. In this study, we describe a novel type II a7 PAM, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744), that exhibits a unique pharmacological profile. In oocytes expressing a7 nAChRs, A-867744 potentiated acetylcholine (ACh)-evoked currents, with an EC50 value of ~1 µM. At highest concentrations of A-867744 tested, ACh-evoked currents were essentially nondecaying. At lower concentrations, no evidence of a distinct secondary component was evident in contrast to 4-naphthalen-1-yl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonic acid amide (TQS), another type II a7 PAM. In the presence of A-867744, ACh concentration responses were potentiated by increases in potency, Hill slope, and maximal efficacy. When examined in rat hippocampus CA1 stratum radiatum interneurons or dentate gyrus granule cells, A-867744 (10 µM) increased choline-evoked a7 currents and recovery from inhibition/desensitization, and enhanced spontaneous inhibitory postsynaptic current activity. A-867744, like other a7 PAMs tested [1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro-5-trifluoromethyl-phenyl)urea (NS1738), TQS, and 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea (PNU-120596)], did not displace the binding of [3H]methyllycaconitine to rat cortex a7* nAChRs. However, unlike these PAMs, A-867744 displaced the binding of the agonist [3H](1S,4S)-2,2-dimethyl-5-(6-phenylpyridazin-3-yl)-5-aza-2-azoniabicyclo[2.2.1]heptane (A-585539) in rat cortex, with a Ki value of 23 nM. A-867744 neither increased agonist-evoked responses nor displaced the binding of [3H]A-585539 in an a7/5-hydroxytryptamine3 (a7/5-HT3) chimera, suggesting an interaction distinct from the a7 N terminus or M2-3 loop. In addition, A-867744 failed to potentiate responses mediated by 5-HT3A or a3ß4 and a4ß2 nAChRs. In summary, this study identifies a novel and selective a7 PAM showing activity at recombinant and native a7 nAChRs exhibiting a unique pharmacological interaction with the receptor.
Price info:
|
1 MG |
40 EUR |
|
5 MG |
80 EUR |
|
10 MG |
110 EUR |
|
300uL of 10mM solution |
60 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


