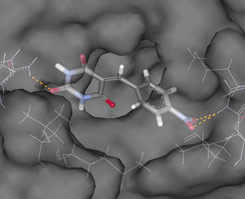
 | Ligand in ER active site.
Docking example from OTAVAchemicals Estrogen Receptor focused library.
The detection of H-bonding between ligand and key ER residues (His524 and Arg394) was used for the library preparation.
|
The sex steroid hormone estrogen is important in both men and women for a variety of physiologic processes. Nearly all of the effects of estrogens are mediated through their binding to nuclear proteins called estrogen receptors (ER), transcription factors that regulate expression of estrogen-responsive genes. Other natural compounds and synthetic drugs are also capable of binding to the ER. Some of these compounds mimic the effects of estrogen; others have more antiestrogenic activity.
The recognition that certain ligands can modulate ER in different ways has led to an explosion in the development of new drugs tailored to have specific and selective effects on ER function. These drugs, of which tamoxifen is the prototype, are now collectively known as selective estrogen receptor modulators (SERMs). SERMs can be conveniently divided into three major categories: (1) triphenylethylene derivatives like tamoxifen, (2) other nonsteroidal compounds, and (3) steroidal compounds that have more complete antiestrogenic activity. It is now clear that SERMs with activities ranging from nearly full estrogenic activity to almost pure antiestrogenic activity can be developed for specific therapeutic uses ranging from treatment and prevention of osteoporosis to the prevention and/or treatment of breast cancer. These drugs offer great promise for the development of optimal hormone replacement therapy for women, an approach that might avoid stimulation of the breast while, at the same time, would effectively treat menopausal symptoms, reduce postmenopausal bone loss, and, perhaps, reduce the risk of cardiac disease.
The focused libraries, including docking scores and drug-like properties, are available on request. Feel free to contact us!
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS

