|

In recent years, structured RNAs have become tractable drug targets, enabling the discovery of selective modulators for riboswitches, long noncoding RNAs, G-quadruplexes, and RNA–protein complexes.
Breakthrough advances in library design, screening methodologies, and AI-based prioritization have expanded the scope of RNA-targeted drug discovery (Kovachka et al., Nat Rev Chem 2024; Lundquist et al., SLAS Discovery 2025; Morishita, ChemMedChem 2022; Momentum Bio, 2024).
Modern RNA-focused screening now combines:
-
SPR/BLI for kinetic profiling and specificity ranking,
-
AI-guided virtual enrichment to prioritize compounds with high “RNA-likeness” before experimental testing (Graff et al., Chem Sci 2020; Cao et al., Nat Mach Intell 2023).
OTAVA’s RNA-Binding Library (AI-Optimized) is a focused collection of 662 compounds designed to probe small-molecule interactions with structured RNA motifs.
The set integrates AI-driven cheminformatics, physicochemical optimization, and scaffold-level clustering to ensure a balanced representation of RNA-privileged chemotypes suitable for high-throughput screening.
Library Design and Optimization
The AI-optimization workflow was applied to OTAVA’s in-stock collection to generate an enriched RNA-targeted subset:
-
Physicochemical filtering
-
pKa-balance filtering
-
PAINS/REOS exclusion
-
Murcko scaffold clustering (Tanimoto ≥ 0.75) using Butina algorithm.
-
AI-based desirability scoring (RepScore) combining TPSA, LogP, flexibility, and QED.
Result: 662 representative compounds covering unique Murcko scaffolds, ensuring broad yet nonredundant coverage of RNA-relevant chemical space.
The AI-optimized core of 662 representative compounds can be readily expanded to approximately 971 structures by including all pre-clustered analogs prior to Murcko filtering, and further extended to about 2,368 predicted candidates generated through Bayesian modeling–guided enrichment of RNA-privileged chemotypes.
Scaffold Diversity Overview
The AI-filtered RNA library retains a structurally rich distribution of scaffolds typical for RNA-binding chemotypes.
Most frameworks contain 9–25 analogs each, with an average molecular weight of ~340 Da and cLogP 2.9–4.5 — parameters consistent with experimentally validated RNA ligands.
|
Murcko |
n_compounds |
n_clusters |
avg_MW |
avg_LogP |
avg_TPSA |
representatives |
|
O=C(Nc1ccccc1)c1cc2cccnc2s1 |
|
25 |
11 |
348.879160 |
4.136057 |
81.680800 |
1 |
|
O=C(Nc1ccccc1)c1ccc(-c2ccccc2)o1 |
21 |
18 |
362.971286 |
4.766846 |
72.026190 |
1 |
|
O=S1(=O)C[C@H](NCCc2ccccc2)[C@@H](S(=O)(=O)c2c... |
14 |
6 |
459.462000 |
1.227423 |
115.283571 |
1 |
|
c1ccc(-c2ccc3ccccc3n2)cc1 |
13 |
9 |
356.079615 |
2.950908 |
86.564615 |
1 |
|
c1ccc2ncc(CN3CCNCC3)cc2c1 |
11 |
7 |
309.284182 |
2.640735 |
41.028182 |
1 |
|
O=C(Nc1ccccc1)c1cc2ccccc2o1 |
11 |
11 |
291.299000 |
4.237509 |
54.169091 |
1 |
|
c1ccc2ncc(CN3CCCCC3)cc2c1 |
10 |
6 |
322.545200 |
4.063380 |
45.775000 |
1 |
|
c1ccc2[nH]cnc2c1 |
10 |
9 |
221.356900 |
2.127286 |
48.704000 |
1 |
|
O=C(Nc1ccccc1)Nc1ccccc1 |
10 |
8 |
290.839100 |
3.773536 |
62.031000 |
1 |
|
O=c1cc[nH]c2ccccc12 |
9 |
9 |
235.652444 |
1.321258 |
82.007778 |
1 |
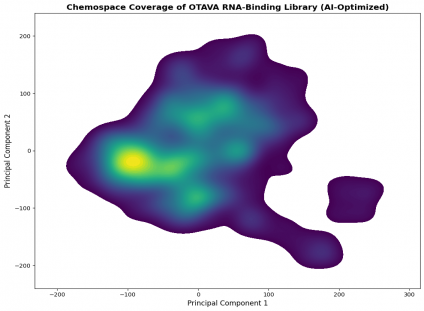
Chemospace Coverage
Principal-component analysis (PCA) of ECFP4 fingerprints confirms even and continuous coverage of the RNA-relevant chemical space.
The library features one dense, drug-like core (TPSA 60–90, cLogP 1.5–4.5) and smaller peripheral clusters representing alternative heteroaromatic scaffolds.
This ensures both exploratory breadth and focused screening efficiency.
All the compounds are in stock, cherry-picking is available.
The libraries (DB, SD, XLS, PDF format) as well as the price-list are available on request. Feel free to contact us or use on-line form below to send an inquiry if you are interested to obtain this library or if you need more information.
Request Your Library Today! Fill out the form:
References:
1. Bernat, V., & Disney, M. D. (2015). RNA structures as mediators of neurological diseases and as drug targets. Neuron, 87(1), 28–46. https://doi.org/10.1016/j.neuron.2015.06.012
2. Thomas, J. R., & Hergenrother, P. J. (2008). Targeting RNA with small molecules. Chemical Reviews, 108(4), 1171–1224. https://doi.org/10.1021/cr0681546
3. Chen, J. L., & Miller, B. G. (2020). Tools for studying small molecule–RNA interactions in vitro and in cells. Biopolymers, 112(3), e23391. https://doi.org/10.1002/bip.23391
4. Childs-Disney, J. L., Yang, X., Gibaut, Q. M. R., Tong, Y., Batey, R. T., & Disney, M. D. (2022). Targeting RNA structures with small molecules. Nature Reviews Drug Discovery, 21(10), 736–762. https://doi.org/10.1038/s41573-022-00521-4
5. Fitzgerald, K., Ragunathan, K., & Znosko, B. M. (2025). Using native mass spectrometry to study RNA–ligand interactions. bioRxiv. https://doi.org/10.1101/2025.02.09.637332
6. Hargrove, A. E. (2021). Small molecule–RNA targeting: Starting with the fundamentals. Trends in Pharmacological Sciences, 42(9), 744–757. https://doi.org/10.1016/j.tips.2021.06.006
7. Rosenblum, S. L., Allen, T. E. H., Wicks, S. L., et al. (2024). Live-cell screening to identify RNA-binding small-molecule inhibitors of the pre-let-7–LIN28 RNA–protein interaction. ACS Chemical Biology, 19(3), 659–671. https://doi.org/10.1021/acschembio.3c00877
8. Stanford Office of Technology Licensing. (2023). Sensors for high-throughput screening of RNA-modulating drugs. Stanford TechFinder. https://techfinder.stanford.edu/technology/sensors-high-throughput-screening-rna-modulating-drugs
9. Suresh, B. M., & Disney, M. D. (2021). Affinity-based methods for the discovery of RNA-binding small molecules. ACS Chemical Biology, 16(2), 219–232. https://doi.org/10.1021/acschembio.0c00844
10. Wicks, S. L., Morgan, B. S., Wilson, A. W., & Hargrove, A. E. (2023). Probing bioactive chemical space to discover RNA-targeted small molecules. bioRxiv. https://doi.org/10.1101/2023.07.31.551350
11. Hargrove, A. E. (2020). Small molecule–RNA targeting: Starting with the fundamentals. Chemical Communications, 56(93), 14788–14797. https://doi.org/10.1039/D0CC06796B
12. Ma, Z., Su, H., Yang, Y., et al. (2025). Development of a DNA-encoded library screening method “DEL Zipper” to empower the study of RNA-targeted chemical matter. SLAS Discovery, 31, 100204. https://doi.org/10.1016/j.slasd.2024.100204 (Epub 2024 Dec 21)
13. Lundquist, K. P., Romeo, I., Puglielli, R. B., et al. (2025). Design, synthesis, and screening of an RNA-optimized fluorinated fragment library. SLAS Discovery, 31, 100215. https://doi.org/10.1016/j.slasd.2025.100215
14. Nickbarg, E. B., Spencer, K. B., Mortison, J. D., & Lee, J. T. (2023). Targeting RNA with small molecules: Lessons learned from Xist RNA. RNA, 29(4), 463–472. https://doi.org/10.1261/rna.079523.122
15. Martin, W. J., Grandi, P., & Marcia, M. (2021). Screening strategies for identifying RNA- and ribonucleoprotein-targeted compounds. Trends in Pharmacological Sciences, 42(9), 758–771. https://doi.org/10.1016/j.tips.2021.06.001
16. Morishita, E. C. (2022). Discovery of RNA-targeted small molecules through merging experimental and computational technologies. Expert Opinion on Drug Discovery, 17(12), 1289–1307. https://doi.org/10.1080/17460441.2022.2134852
17. Kovachka, S., Panosetti, M., Grimaldi, B., Azoulay, S., Di Giorgio, A., & Duca, M. (2024). Small molecule approaches to targeting RNA. Nature Reviews Chemistry, 8(2), 120–135. https://doi.org/10.1038/s41570-023-00569-9
18. Graff, D. E., Shakhnovich, E. I., & Coley, C. W. (2020). Accelerating high-throughput virtual screening through molecular pool-based active learning. Chemical Science, 11(47), 12028–12036. https://doi.org/10.1039/D0SC06805E
19. Cao, Z., Sciabola, S., & Wang, Y. (2023). Large-scale pretraining improves sample efficiency of active-learning–based molecule virtual screening. arXiv preprint. http://arxiv.org/pdf/2309.11687v1
20. Tomemori, M., Ichijo, R., Shinohara, Y., Hatta, K., Nakatani, K., & Kawai, G. (2025). NMR analysis of interactions between RNA structure elements and small molecules. Journal of Biochemistry, 178(1), 1–9. https://doi.org/10.1093/jb/mvaf020
21. Momentum Bio. (2024). Screening strategies for RNA-targeted small molecule drugs. Momentum Bio. https://momentum.bio/content/screening-strategies-for-rna-targeted-small-molecule-drugs/
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS



