|
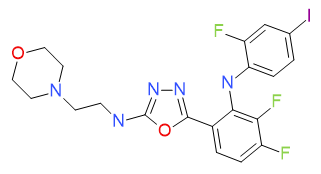
5-{3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl}-N-(2-morpholin-4-ylethyl)-1,3,4-oxadiazol-2-amine
MEK1 inhibitor (IC50 = 532 mM)
Chemical Formula: C20H19F3IN5O2
Molecular Weight: 545.3063
OTAVAchemicals Catalogue Number: 7070707138
CAS Registry Number: 548756-68-9
Purity: 99% (HPLC)
Ref. 1 Warmus, J. S., Flamme, C., Zhang, L. Y., Barrett, S., Bridges, A., Chen, H., … Zhang, E. (2008). 2-Alkylamino- and alkoxy-substituted 2-amino-1,3,4-oxadiazoles—O-Alkyl benzohydroxamate esters replacements retain the desired inhibition and selectivity against MEK (MAP ERK kinase). Bioorganic & Medicinal Chemistry Letters, 18(23), 6171–6174.
Ref. 2. Chen, Po-Yuan; Hong, Hong-Jye; Jhuo, Mien-De, et al. Computational screening of novel mitogen-activated protein kinase kinase-1 (MEK1) inhibitors by docking and scoring. Journal of Life Sciences (Libertyville, IL, United States) (2011), 5(6), 434-442
Ref. 3. Ohren, J. F., Chen, H., Pavlovsky, A., Whitehead, C., Zhang, E., Kuffa, P., … Hasemann, C. A. (2004). Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nature Structural & Molecular Biology, 11(12), 1192–1197.
Abstract 1 : This paper reports a second generation MEK inhibitor. The previously reported potent and efficacious MEK inhibitor, PD-184352 (CI-1040), contains an integral hydroxamate moiety. This compound suffered from less than ideal solubility and metabolic stability. An oxadiazole moiety behaves as a bioisostere for the hydroxamate group, leading to a more metabolically stable and efficacious MEK inhibitor.
Abstract 2. The mitogen-activated protein kinase (MAPK) cell signal transduction pathways play a key role in determ ining the survival of cells. If these pathways can be controlled, they will prohibit the proliferation of cancer cells. To attain this goal, the authors utilize many drugs to interact with mitogen-activated protein kinase kinase-1 (MEK1) in MAPK, and use computer aided drug design (C ADD) to analyze the ligand activities of proteins in MEK1. The results show that in these drugs, the aromatic group in the terminal of the protein and the PHE209 will induce the stacking force, which is highly related to the actual activities of these drugs.
Abstract 3 MEK1 and MEK2 are closely related, dual-specificity tyrosine/threonine protein kinases found in the Ras/Raf/MEK/ERK mitogen-activated protein kinase (MAPK) signaling pathway. Approximately 30% of all human cancers have a constitutively activated MAPK pathway, and constitutive activation of MEK1 results in cellular transformation. Here we present the X-ray structures of human MEK1 and MEK2, each determined as a ternary complex with MgATP and an inhibitor to a resolution of 2.4 A and 3.2 A, respectively. The structures reveal that MEK1 and MEK2 each have a unique inhibitor-binding pocket adjacent to the MgATP-binding site. The presence of the potent inhibitor induces several conformational changes in the unphosphorylated MEK1 and MEK2 enzymes that lock them into a closed but catalytically inactive species. Thus, the structures reported here reveal a novel, noncompetitive mechanism for protein kinase inhibition.
DOI:
10.1016/j.bmcl.2008.10.015
10.1038/nsmb859
Price info:
|
1 MG |
59 EUR |
|
5 MG |
99 EUR |
|
10 MG |
129 EUR |
|
300uL of 10mM solution |
79 EUR
|
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS


