|
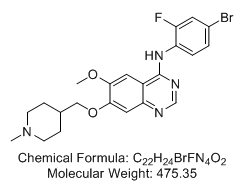 N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-[(1-methyl-4-piperidinyl)methoxy]-4-quinazolinamine (Vandetanib; ZD6474; Zactima) N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-[(1-methyl-4-piperidinyl)methoxy]-4-quinazolinamine (Vandetanib; ZD6474; Zactima)
selectively inhibits the tyrosine kinase activity of vascular endothelial growth factor receptor 2 (VEGF2), thereby blocking VEGF-stimulated endothelial cell proliferation and migration and reducing tumor vessel permeability. This agent also blocks the tyrosine kinase activity of epidermal growth factor receptor (EGFR), a receptor tyrosine kinase that mediates tumor cell proliferation and migration and angiogenesis. It also inhibits RET-tyrosine kinase activity, an important growth driver in certain types of thyroid cancer
OTAVAchemicals Catalogue Number: 1156355
CAS Registry Number: 443913-73-3
Purity: 95%+ (HPLC)
Ref. 1: Hanrahan & Heymach. Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors Vandetanib (ZD6474) and AZD2171 in Lung Cancer. Clinical Cancer Research (2007), 13(15, Pt. 2), 4617s-4622s
A review. Vascular endothelial growth factor (VEGF) is a rational target for advanced non–small cell lung cancer (NSCLC), a hypothesis validated by the recent Eastern Cooperative Oncology Group E4599 trial showing that the addition of the VEGF monoclonal antibody bevacizumab to chemotherapy prolongs overall survival. Several new tyrosine kinase inhibitors targeting the VEGF pathway are currently in advanced clinical development for NSCLC and offer several possible advantages compared with monoclonal antibodies, including oral administration, more flexible dosing, a broader spectrum of target inhibition, and different toxicity profiles. Among these agents, vandetanib (ZD6474), an inhibitor of the VEGF receptor (VEGFR)-2 and epidermal growth factor receptor tyrosine kinase, has been the most extensively studied. In a randomized phase II study of patients with platinum-refractory NSCLC, including squamous histology, vandetanib prolonged progression-free survival compared with gefitinib. In another phase II trial, an improvement in progression-free survival was observed for vandetanib in combination with docetaxel compared with docetaxel alone. AZD2171 is an inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3 and other tyrosine kinases that has shown clinical activity in NSCLC in combination with carboplatin and paclitaxel. Several phase III trials are under way testing these agents either as monotherapy or in combination with chemotherapy in patients with lung cancer. Early results with these agents, and others being tested, raise the possibility that there will eventually be multiple VEGF-targeted therapies available in the clinic that can potentially benefit a broader range of patients with advanced-stage NSCLC.
Ref. 2: Sahtornsumetee & Rich. Vandetanib (ZD6474), a novel multitargeted kinase inhibitor, in cancer therapy. Drugs of Today (2006), 42, 657-670
A review. In clinical trials thus far, single-targeted kinase inhibitors have shown only limited success in demonstrating survival benefits in cancer. This has led to the development of multitargeted kinase inhibitors capable of disrupting various mitogenic pathways in both cancer cells and associated vasculature. Vandetanib is a novel multitargeted kinase inhibitor exhibiting potent activity against vascular endothelial growth factor receptor-2 (VEGFR-2; kinase insert domain-containing receptor [KDR]) and, to a lesser extent, epidermal growth factor receptor (EGFR) and RET kinase. Vascular endothelial growth factor (VEGF) and VEGFR-2 play a pivotal role in regulating angiogenesis and vascular permeability in cancers. In addition to its antiangiogenic effects, vandetanib acts against EGFR, which is overexpressed or mutated in several solid tumors. Furthermore, vandetanib exerts activity against oncogenic RET kinase, the overexpression of which is common in medullary and papillary thyroid carcinomas. Therefore, the multitargeted kinase inhibitor vandetanib represents a new approach, targeting both tumor cells and tumor-associated endothelial cells. Preclinical studies of vandetanib have demonstrated antitumor efficacy against multiple human cancer xenografts in subcutaneous, orthotopic and metastatic models. Phase I clinical trials have demonstrated that vandetanib is well tolerated. Common adverse events included rash, diarrhea and asymptomatic QTc prolongation. Phase II clinical studies in patients with non-small-cell lung cancer have shown promising results, employing vandetanib as both monotherapy and in combination with docetaxel. Phase II studies in other cancers have likewise been initiated. This review summarizes preclinical and clinical studies of vandetanib for the treatment of cancers.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS
 N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-[(1-methyl-4-piperidinyl)methoxy]-4-quinazolinamine (Vandetanib; ZD6474; Zactima)
N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-[(1-methyl-4-piperidinyl)methoxy]-4-quinazolinamine (Vandetanib; ZD6474; Zactima)