|
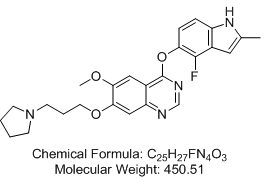 4-[(4-Fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline (AZD2171; Cediranib) 4-[(4-Fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline (AZD2171; Cediranib)
an oral tyrosine kinase inhibitor of all of the VEGF receptors (VEGFR1, VEGFR2, VEGFR3), as well as KIT and (less potently) PDGFRA and PDGFRB
OTAVAchemicals Catalogue Number: 1156354
CAS Registry Number: 288383-20-0
Purity: 95%+ (HPLC)
Ref. 1: Wedge et al. AZD2171: A Highly Potent, Orally Bioavailable, Vascular Endothelial Growth Factor Receptor-2 Tyrosine Kinase Inhibitor for the Treatment of Cancer. Cancer Research (2005), 65, 4389-4400
Abstract: Inhibition of vascular endothelial growth factor-A (VEGF) signaling is a promising therapeutic approach that aims to stabilize the progression of solid malignancies by abrogating tumor-induced angiogenesis. This may be accomplished by inhibiting the kinase activity of VEGF receptor-2 (KDR), which has a key role in mediating VEGF-induced responses. The novel indole-ether quinazoline AZD2171 is a highly potent (IC50 <1 nmol/L) ATP-competitive inhibitor of recombinant KDR tyrosine kinase in vitro. Concordant with this activity, in human umbilical vein endothelial cells, AZD2171 inhibited VEGF-stimulated proliferation and KDR phosphorylation with IC50 values of 0.4 and 0.5 nmol/L, respectively. In a fibroblast/endothelial cell coculture model of vessel sprouting, AZD2171 also reduced vessel area, length, and branching at subnanomolar concentrations. Once-daily oral administration of AZD2171 ablated experimental (VEGF-induced) angiogenesis in vivo and inhibited endochondral ossification in bone or corpora luteal development in ovary; physiologic processes that are highly dependent upon neovascularization. The growth of established human tumor xenografts (colon, lung, prostate, breast, and ovary) in athymic mice was inhibited dose-dependently by AZD2171, with chronic administration of 1.5 mg per kg per day producing statistically significant inhibition in all models. A histologic analysis of Calu-6 lung tumors treated with AZD2171 revealed a reduction in microvessel density within 52 hours that became progressively greater with the duration of treatment. These changes are indicative of vascular regression within tumors. Collectively, the data obtained with AZD2171 are consistent with potent inhibition of VEGF signaling, angiogenesis, neovascular survival, and tumor growth. AZD2171 is being developed clinically as a once-daily oral therapy for the treatment of cancer.
http://cancerres.aacrjournals.org/cgi/reprint/65/10/4389.pdf
Ref. 2: Sorbera et al. Cediranib. Drugs of the Future (2007), 32, 577
Abstract: Angiogenesis is a complex biological event in which vascular endothelial growth factor (VEGF) is considered the rate-limiting step. VEGF mediates both physiological and pathological angiogenesis via binding to specific transmembrane receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR or Flk-1), expressed mainly on vascular endothelial cells. Because angiogenesis in healthy adults is generally absent, interruption of VEGF signaling is an attractive strategy to selectively inhibit angiogenesis in solid tumors. Antagonism of VEGFR-2 has attracted particular attention due to the generally limited expression of this receptor in endothelium and the crucial role it plays in VEGF-mediated angiogenic signaling. Cediranib (AZD-2171, Recentin) is a novel, orally available quinazoline VEGFR inhibitor that was shown to potently inhibit VEGFR-1, VEGFR-2 and VEGFR-3 tyrosine kinase activity and VEGF-mediated signaling in vitro and in vivo. Cediranib exerted marked anticancer effects in vivo in a variety of xenograft models and in patients with advanced solid tumors. It continues to undergo clinical testing alone and in combination with selected chemotherapies for the oral treatment of various cancers.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS
 4-[(4-Fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline (AZD2171; Cediranib)
4-[(4-Fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline (AZD2171; Cediranib)